Artificial intelligence is transforming the world of clinical trials, promising to cut drug development timelines in half. A recent white paper from Artefact explores this ongoing revolution, highlighting innovations reshaping every stage of the process, from trial design to patient recruitment.
The paper offers insight into a dynamic ecosystem where startups, tech giants, and pharmaceutical labs are redefining the future of medical research.
How AI is reshaping the clinical trial landscape: an analysis by Artefact
The pharmaceutical industry is on the brink of a major revolution in clinical trials, fueled by artificial intelligence (AI). A recent white paper from Artefact, in partnership with AI for Health, explores how AI is transforming every stage of the clinical trial process, from design to result analysis.
An unprecedented opportunity to accelerate drug development
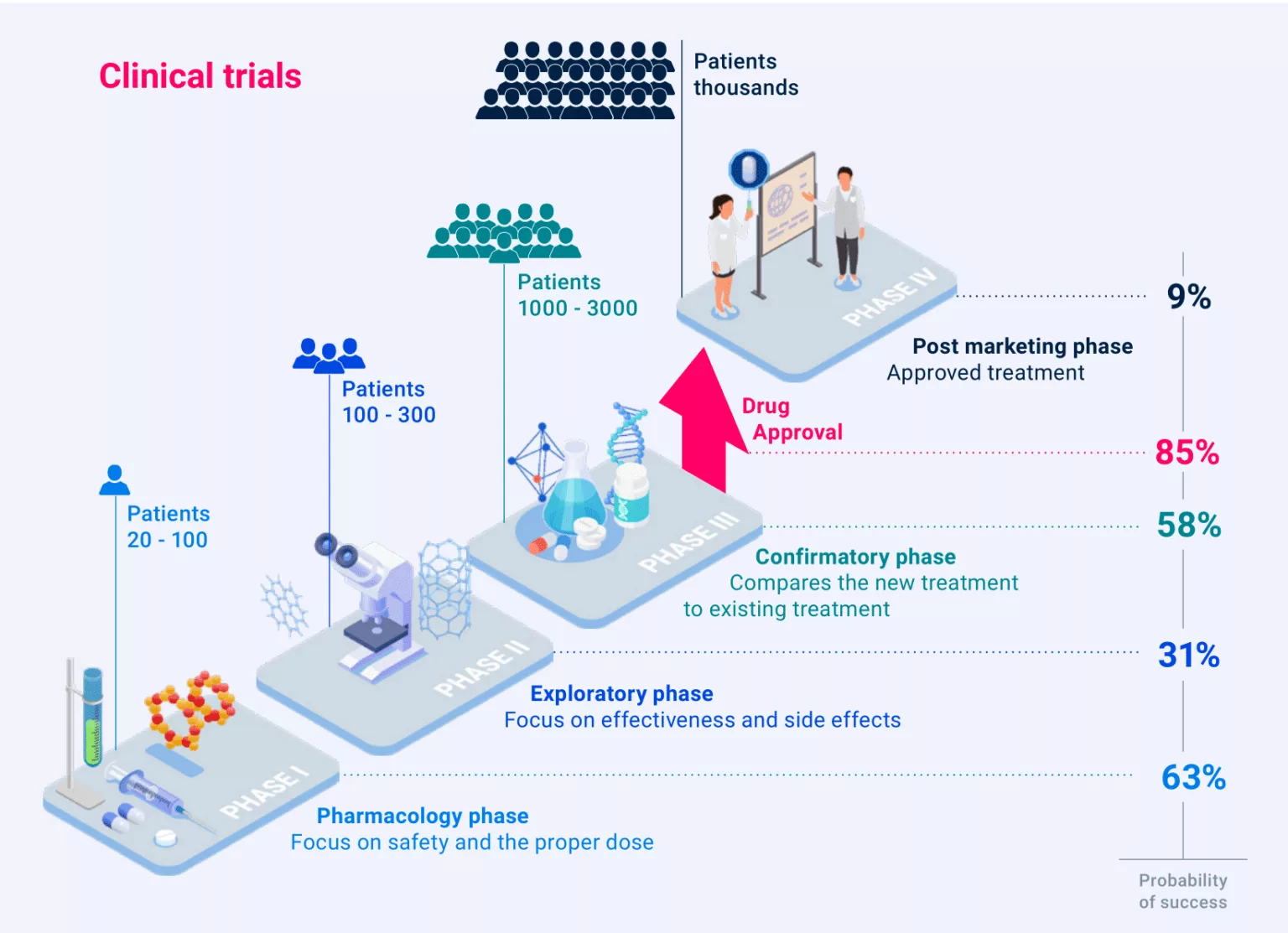
In a sector where failure is the norm – with 9 out of 10 drug candidates failing during clinical trials – AI presents a revolutionary opportunity. By reducing development timelines by several weeks, AI has the potential to save millions for pharmaceutical companies, accelerate scientific breakthroughs, and bring life-saving therapies to patients faster than ever before.
The current failure rate of clinical trials for new drugs, from phase I to final clinical approval, exceeds 90%. The main reasons for these failures include lack of clinical effectiveness (40-50%), unmanageable toxicity (30%), poor drug properties (10-15%), and absence of commercial needs or poor strategic planning (10%).
“We chose to focus on AI and generative AI in pharma R&D for several reasons. First, it’s gaining significant interest and acceleration from many stakeholders. Second, AI and R&D are crucial strategic topics today, with the potential to halve clinical trial durations. Finally, it’s a very current topic, as many laboratories are already deploying or planning to activate these use cases.”says Thomas Filaire, supervisor of the white paper at Artefact to explains the motivations behind the study
AI is redefining each step of the clinical trial life cycle.
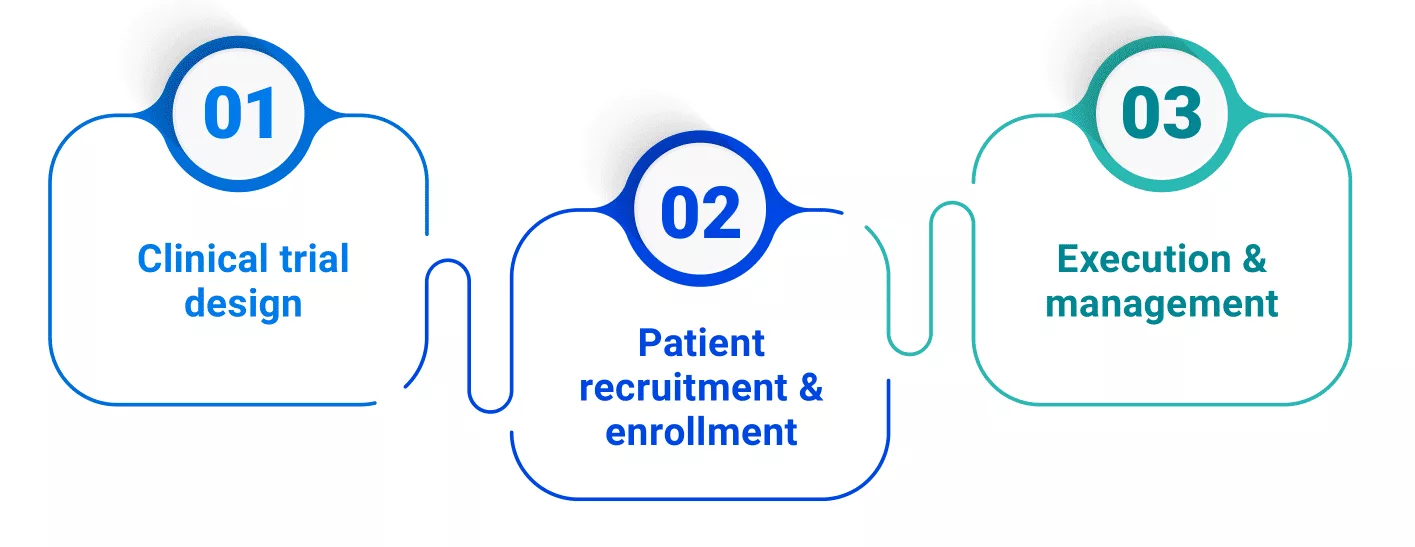
Artefact’s white paper explores how AI is revolutionizing the three key phases of clinical trials:
1. Clinical Trial design
AI optimizes the traditionally long and complex process of designing clinical trial protocols. Key innovations include:
These advancements significantly reduce the trial design time, sometimes cutting it from several months to just weeks. For example:
2. Patient recruitment and inclusion
Recruiting patients remains one of the main challenges in clinical trials. AI offers innovative solutions to:
The impact of AI on recruitment is significant:
3. Execution and management of clinical trials
AI is transforming clinical trial execution and analysis through several advancements:
These innovations drive efficiency, reduce costs, and streamline the entire clinical trial process, speeding up drug development and improving outcomes.
These innovations are reshaping the clinical trial landscape, making them faster, more efficient, and cost-effective.
An expanding innovation ecosystem.
The white paper highlights the pivotal role played by various stakeholders in advancing AI solutions for clinical trials, with a special focus on innovative startups and leading tech giants.
The leading tech giants driving innovation.
Tech giants like Google, Microsoft, IBM, and Apple are playing an increasingly vital role in advancing AI-driven clinical trials:
“We are moving from a reactive healthcare ecosystem to a proactive, almost predictive one.”emphasizes Shweta Maniar, Global Director of Health and Life Sciences at Google Cloud
This shift reflects the transformative impact of AI on the entire clinical trial process.
Startups, driving innovation in clinical research.
Many startups are emerging in the clinical research field, offering innovative solutions for trial design, patient recruitment, and data management. The white paper outlines a map of these innovative players, organized according to the three key phases of clinical trials:
1. Clinical Trial design:
2. Patient recruitment and inclusion:
“Less than 5% of patients benefit from oncology clinical trials, while 70% say they would be willing to participate if given the opportunity. There’s a clear need for better matching between patients and trials, and recent AI advancements make this possible.”highlights as a critical issue Thomas Peyresblanques, co-founder and CEO of Klineo
3. Execution and management of trials:
“With only 4% of trials including a representative population, Inato helps sponsors recruit patients twice as fast, increasing diversity to 67% non-white participants, compared to a previous average of 15%.”explains Kourosh Davarpanah, co-founder and CEO of Inato, highlighting the impact of their solution
This reflects the potential of AI to improve recruitment and inclusion in clinical trials.
Challenges to overcome
Despite the promising advancements, challenges remain regarding the adoption of AI in clinical trials:
In conclusion, AI offers unprecedented opportunities to revolutionize clinical trials, but widespread adoption requires addressing these complex challenges. Collaboration between startups, tech giants, pharmaceutical companies, and regulators will be essential to fully harness AI’s potential while ensuring the safety and ethics of clinical trials.
(1) : Zhang, B., Zhang, L., Chen, Q. et al. Harnessing artificial intelligence to improve clinical trial design. Commun Med 3, 191 (2023). https://doi.org/10.1038/s43856-023-00425-3
(2) : Tsuchiwata S, Tsuji Y. Computational design of clinical trials using a combination of simulation and the genetic algorithm. CPT Pharmacometrics Syst Pharmacol. 2023 Apr;12(4):522-531. doi: 10.1002/psp4.12944. Epub 2023 Mar 5. PMID: 36793239; PMCID: PMC10088085.
(4) : Ismail A, Al-Zoubi T, El Naqa I, Saeed H. The role of artificial intelligence in hastening time to recruitment in clinical trials. BJR Open. 2023 May 16;5(1):20220023. doi: 10.1259/bjro.20220023. PMID: 37953865; PMCID: PMC10636341.

 BLOG
BLOG